Redirected from Hydrogen Assisted Cracking
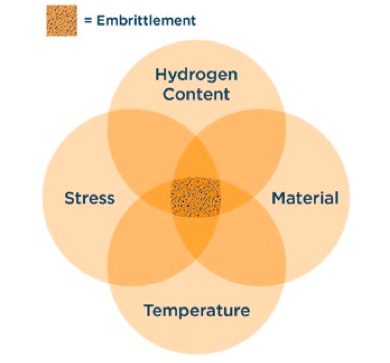
Hydrogen Embrittlement is a form of hydrogen damage stemming from the diffusion of atomic hydrogen into certain types of metals. There are three criteria that lead to hydrogen embrittlement:
- Hydrogen,
- High strength steels and alloys, and
- Applied or residual stress.
In the presence of hydrogen, these high strength materials lose their ductile properties and become vulnerable to brittle fracture. Crack initiation and propagation can occur internally, however, most cases are present on the surface of a component. Even low concentrations of hydrogen can have detrimental effects on equipment.
Hydrogen embrittlement primarily occurs in ferrous alloys such as high strength and martensitic steels. Generally, the greater the strength of the material the more vulnerable it is to hydrogen embrittlement. Other materials, including some copper, aluminum, and nickel alloys, have low susceptibility to this type of hydrogen damage. However, if these materials have undergone a strain hardening process, they may be at greater risk for embrittlement.
Hydrogen embrittlement is a common occurrence in the oil & gas and petrochemical industries. Process equipment and units that are commonly affected include:
- Carbon steel piping
- Fluid catalytic cracking units
- Hydrotreaters
- Hydrocrackers
- Storage tanks
- Reactors
- Coke drums
- HF alkylation units
Other parts, such as bolts and springs have high strengths to withstand large loads and stresses. Because of their high tensile strengths, these parts are subject to cracking.
There are several hydrogen damage mechanisms related to hydrogen embrittlement. These mechanisms include:
Although hydrogen embrittlement is associated with cracking, it can also be associated with corrosion damage in some cases.
Where Does the Hydrogen Come From?
The diffusion of hydrogen can happen during many processes at low temperatures as well as high temperatures. Welding practices generally induce heat affected zone (HAZ) areas between the filler metal and parent metal. These HAZs can be critical points of hydrogen damage even if the parent metal is resistant to embrittlement. Pickling (i.e., removing thick oxide scale) is also a process that may lead to corrosive damage due to the acidic solutions used to clean the surface of the component. Hydrogen atmospheres at high temperatures as well as wet H2S and HF acid services, also accelerate hydrogen embrittlement damage.
One key difference between hydrogen embrittlement and stress corrosion cracking, although very similar in many aspects, is their reaction to cathodic protection. Cathodic protection is one of the most effective methods of corrosion protection, however, it does not prevent the diffusion of hydrogen into the steel component.
Prevention
Several techniques can be employed to reduce the likelihood of hydrogen embrittlement.
Material Selection
In some cases, lower strength steels and alloys can be used to decrease the risk of embrittlement. However, special considerations must be taken into account to ensure the material can withstand the applied loads during operation. If high strength steels and alloys are the best material choice, certain heat treatments can be performed to reduce the hardness and residual stresses that may lead to embrittlement issues.
Hydrogen Bake-Out (dehydrogenation)
Hydrogen bake-out is a common industry practice used to drive hydrogen out of the steel. This is especially important for weldments as trapped hydrogen can cause brittle fracture. The process involves heating the steel to roughly 600℉ (316℃) and soaking for a certain period of time (depending on the size of the component and thickness of material). Soaking steels at higher bake-out temperatures ensures that a sufficient amount of hydrogen has been removed before repairs can be made. The limitation to this technique is determining the amount of time the steel needs to be soaked. A general rule of thumb is one hour per inch of material thickness.
Post Weld Heat Treatment (PWHT)
PWHT is usually performed shortly after welding to reduce residual stresses and control the temperature fluctuation between welding and operating conditions.
Inspection & Monitoring
Inspection — The most useful and cost-effective inspection methods used to identify hydrogen embrittlement cracking are magnetic particle testing (MPT) and ultrasonic testing (UT). MPT is particularly useful for identifying surface cracks. UT techniques can also accurately locate crack on the surface or sub-surface of the component.
Monitoring — Hydrogen flux monitors resolves the hydrogen bake-out issue of uncertainty about the amount of time a steel needs to be soaked. These monitors are used during hydrogen bake-out to determine when the hydrogen flux rate through the steel drops below or rises above a certain threshold. Above this threshold, hydrogen bake-out is initiated and continues until the hydrogen flux rate drops below the threshold. These monitors determine when bake-outs can be shortened or extended.
Relevant Codes and Standards
API RP 571 — Damage Mechanisms Affecting Fixed Equipment in the Refining Industry
Related Topics
- Brittle Fracture
- Carburization
- Cavitation
- CO2 Corrosion
- Cooling Water Corrosion
- Corrosion Fatigue
- Corrosion Under Insulation (CUI)
- Cracking
- Decarburization
- Embrittlement
- Erosion Corrosion
- Fatigue (Material)
- Flue Gas Dew Point Corrosion
- Graphitization
- Green Rot
- High Temperature Hydrogen Attack (HTHA)
- High-Temperature Creep
- Hydrochloric (HCl) Acid Corrosion
- Hydrofluoric (HF) Acid Corrosion
- Hydrogen Stress Cracking
- Liquid Metal Embrittlement (LME)
- Metal Dusting
- Microbiologically Influenced Corrosion (MIC)
- Naphthenic Acid Corrosion (NAC)
- Phosphoric Acid Corrosion
- Pitting Corrosion
- Spheroidization (Softening)
- Stress Assisted Corrosion
- Sulfidation Corrosion
- Sulfuric Acid Corrosion
- Thermal Fatigue
- Vibration-Induced Fatigue
- Wet H2S Damage
Relevant Links
Topic Tools
Share this Topic
Contribute to Definition
We welcome updates to this Integripedia definition from the Inspectioneering community. Click the link below to submit any recommended changes for Inspectioneering's team of editors to review.
Contribute to Definition