Hydrogen Induced Cracking (HIC) is a common form of wet H2S damage caused by the initiation and propagation of small crack-like flaws, often resembling small internal voids or blisters, that are generally laminar (in-plane) and oriented parallel to the inside and outside surfaces of steel. HIC usually occurs due to the effects of aqueous hydrogen diffusing into steel in wet H2S refinery process environments. It can occur at relatively low temperatures, largely as a result of atomic hydrogen from wet H2S corrosion reactions which enter the steel and collect at inclusions or impurities within the steel. The H2S prevents the hydrogen recombination reaction that would normally occur, so rather than bubbling off from the corroding surface, the hydrogen atoms are forced into the metal structure causing corrosion and weakness.
As far as damage mechanisms go, HIC is usually, but not always, fairly innocuous. It typically isn’t damaging until it becomes extensive and affects material properties. Over time, these cracks tend to link-up due to internal pressure build-up and possibly local stress fields in damaged regions, often propagating into a weld or progressing in the through-thickness direction (a.k.a “stepwise” cracking).
Areas Susceptible to Hydrogen Induced Cracking
Many variables dictate the probability of experiencing in-service wet H2S damage, including HIC. Furthermore, there are many different nuances associated with process conditions/contaminants, welding/steel making practices, applied/residual stress fields, etc., that promote different forms of wet H2S cracking or blistering.
Several refinery process units are distinctly prone to wet H2S damage like HIC, including but not limited to, fluid catalytic cracking units (FCCUs), amine units, sulfur recovery units, and sour water stripper units. In FCCUs, light ends recovery/fractionation (overhead) equipment and especially locations downstream of wet gas compressors, intercoolers/aftercoolers, knock-out drums, sponge absorbers, and deethanizers have historically been problem areas. Amine treating unit absorbers/contactors (especially near sour gas inlet nozzles) and sour water stripper unit overhead equipment (e.g., condensers and reflux drums) have also been particularly susceptible.
Detecting Hydrogen Induced Cracking
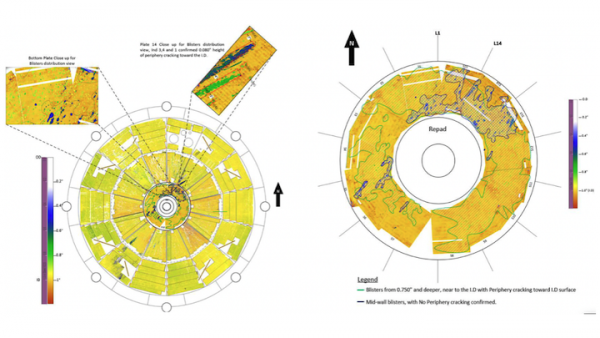
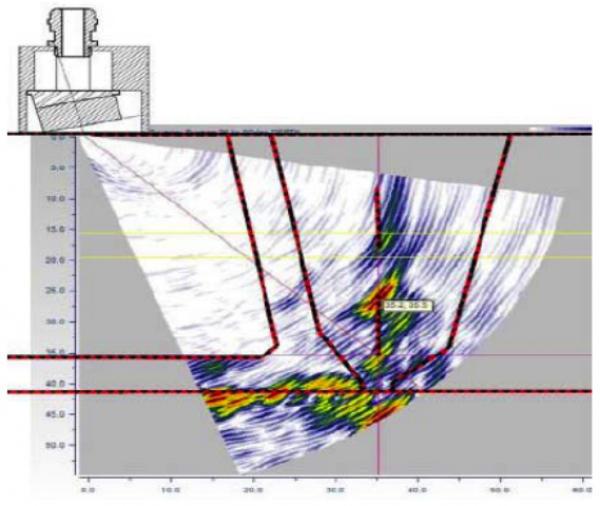
On the surface, HIC is often horseshoe shaped and no bigger than the cuticle of your small finger. Regular inspection and testing should be performed in order to eliminate the chances of hydrogen corrosion. The conventional non-destructive testing (NDT) method for detecting wet H2S cracking is Wet Fluorescent Magnetic Particle Testing (WFMPT) which is sensitive enough to reliably detect surface breaking cracks in the steel caused by HIC. Ultrasonic testing methods like shear wave (SWUT), phased array (PAUT) techniques, total focusing method (TFM), full matrix capture (FMC), time of flight diffraction (TOFD) have also proven effective for categorizing and sizing wet H2S damage like HIC.
Regardless of the NDT method used, it is essential that the limitations and margin of error be well-understood, and that qualified and experienced inspectors are involved. Again, given the possibly unpredictable or sudden damage progression associated with wet H2S damage mechanisms, it is important to ensure that high-risk equipment, and especially regions of previously identified damage, are routinely monitored.
References
- Prueter, P., 2021, “Damage Control: Wet H2S Damage Detection,” Inspectioneering Journal, 27(5), pp. 45-52.
Related Topics
Relevant Links
Topic Tools
Share this Topic
Contribute to Definition
We welcome updates to this Integripedia definition from the Inspectioneering community. Click the link below to submit any recommended changes for Inspectioneering's team of editors to review.
Contribute to Definition