Environment-Assisted Cracking
Amine Stress Corrosion Cracking
| Foundational Concepts |
| Stress Corrosion Cracking |
Amine Stress Corrosion Cracking (or, more commonly, “amine cracking”) is a form of alkaline stress corrosion cracking that affects carbon and low alloy steels. This damage mechanism is associated with lean amine, whereas cracking in rich amine is actually wet H2S cracking.
Critical factors for this damage mechanism are the level of tensile stress (residual stresses from welding, cold working, or fabrication that have not been removed through proper PWHT), amine concentration, and temperature.
Amine cracking occurs in MEA and DEA services, but can also be found in MDEA and DIPA. Cracking has occurred down to ambient temperatures with some amines.
Equipment handling lean amine solutions that has not been stress-relieved or may have been inadequately stress-relieved. This includes contactors, absorbers, strippers, regenerators, and accumulators, as well as any equipment that is subject to inadvertent amine carry over or steam cleaning in preparation for maintenance.
Amine cracking propagates parallel to the weld in adjacent base metal but it can also occur in the weld deposit or heat affected zone (HAZ). Cracks are similar in appearance to those caused by wet H2S damage.
Post-weld heat treatment (PWHT) is an effective way to mitigate amine cracking. Solid or clad stainless steel, alloy 400, or other corrosion resistant alloys can be used in place of carbon steel to provide resistance to amine cracking. For crack detection, performing WFMT, EC, RT, or ACFM is most effective. PT is not effective. AET can be used for monitoring crack growth and locating growing cracks.
Ammonia Stress Corrosion Cracking
| Foundational Concepts |
| Stress Corrosion Cracking |
Ammonia Stress Corrosion Cracking is a form of stress corrosion cracking that commonly occurs in brass tubes in cooling water service that has been contaminated with ammonia due to biological growths or other contamination. This cracking can also occur when ammonia is intentionally added to process streams as a neutralizer by someone unaware of its potential effect on brass tubes. Brass condenser tubes can undergo brittle fracture if bent when significant ammonia stress corrosion cracking is present.
Ammonia stress corrosion cracking can also affect carbon steel equipment, but unlike the cracking mechanism on brass which occurs in an aqueous solution, the cracking of steel equipment occurs in anhydrous ammonia. Systems with air/oxygen contamination also tend to be particularly vulnerable to this issue. Thankfully though, there are several ways in which ammonia stress corrosion cracking can be prevented in steel equipment. First and foremost, systems that have not undergone postweld heat treatment (PWHT) are much more susceptible, thus proper PWHT is essential. Adding a very small amount of water (0.2%) to the anhydrous ammonia can also inhibit the cracking of steel.
When inspecting for ammonia stress corrosion cracking in brass tubes, some of the best techniques to involve the use of eddy current, such as pulsed eddy current testing or eddy current array testing. To inspect for ammonia cracking in steel tubes, wet fluorescent magnetic particle testing is best for where access to the surface is available; when it’s not, shear wave ultrasonic testing tends to work best.
Cupro-nickel alloys are usually not susceptible to ammonia stress corrosion cracking, so if all else fails, upgrading to a new material is another form of prevention. Austenitic stainless steels likewise are resistant to this form of corrosion, so using them is another option.
Carbonate Stress Corrosion Cracking
| Foundational Concepts |
| Stress Corrosion Cracking |
Carbonate Stress Corrosion Cracking (“carbonate cracking”) is a form of alkaline stress corrosion cracking that affects carbon and low alloy steels in “wet” carbonate service. It often occurs more aggressively at higher pH and higher concentrations of carbonate solutions.
Carbonate cracking requires the presence of water in order to form. Most failures have occurred when the pH of the sour water ranges between 8 to 10. Some amount of H2S may be present, although there is no established threshold amount. Furthermore, Ammonia (NH3) raises the pH and therefore increases cracking likelihood.
In FCCUs, higher levels of Feed Nitrogen and lower levels of Feed Sulfur are associated with cracking susceptibility. In most cases, the Feed Nitrogen to Sulfur ratio ranges from 0 to 70.
Carbonate cracking has been observed in the overhead system of FCCUs (where water exists), in SWS strippers, and in Catacarb and CO2 removal facilities of hydrogen manufacturing units. Cracks propagate parallel to the weld in adjacent metal but can also occur in the weld deposit or HAZ. Cracks may be mistaken for SSC or SOHIC, although they are usually further from the toe of the weld and have multiple parallel cracks.
Effective PWHT is usually a good way to prevent Carbonate Cracking, but if it isn’t done right with sufficient soak time and higher temperatures than normal, then cracking may reappear in replacement equipment. Alloying up or applying effective barrier coatings to protect the steel can also be effective. For crack detection, performing WFMT or ACFM is most effective. PT is not effective. AET can be used for monitoring crack growth and locating growing cracks.
Caustic Stress Corrosion Cracking
| Foundational Concepts |
| Stress Corrosion Cracking |
Caustic Stress Corrosion Cracking is a form of alkaline stress corrosion cracking. It is sometimes incorrectly referred to as Caustic Embrittlement; however, no embrittlement is actually occurring with this damage mechanism. With caustic stress corrosion cracking, cracks propagate parallel to the weld in the adjacent base metal but can also occur in the weld deposit or HAZ
Caustic environments cause this type of cracking most commonly in weldments because of high residual stresses, but base metals with high residual stresses can also be affected. Affected materials include carbon steel, low alloy steel, and austenitic stainless steel (nickel-based alloys are more resistant). Caustic stress corrosion cracking can be found in piping and equipment that handles caustic, including H2S and mercaptan removal units. It is also found in equipment that uses caustic for neutralization in sulfuric acid alkylation units and HF alkylation units.
For caustic stress corrosion cracking, proper PWHT of carbon steel, repair welds, and internal and internal attachment welds is an effective way to prevent this from occurring. For crack detection, performing WFMT, EC, RT, or ACFM is most effective. PT is not effective. AET can be used for monitoring crack growth and locating growing cracks.
Chloride Stress Corrosion Cracking
| Foundational Concepts |
| Stress Corrosion Cracking |
Chloride Stress Corrosion Cracking (Cl-SCC) is a type of Stress Corrosion Cracking (SCC) and is one of the most well known forms of SCC in the refining and chemical processing industries. It can be detrimental to austenitic stainless steels, one of the main reasons these steels are not considered a cure-all for corrosion problems. Damage due to Cl-SCC is easily identifiable by the telltale spiderwebbed and lightning-array type network of highly branched cracks.
Despite the facts that we know much about this mechanism and there have been many failures due to it in the past, it continues to plague the industry. This is typically due to inadvertent contamination of equipment with chlorides that was not anticipated by design engineers who are unaware of the potential consequences of using austenitic stainless steels where chlorides may be present.
Fortunately, catastrophic failures from Cl-SCC are rare because of the very high toughness of stainless steel - although they can occur. The consequences from most leaks tend to be economic in nature, although this can still be devastating to some plants due to the high costs associated with replacing equipment.
- Chloride cracking of 300 series stainless steels continues to occur in a number of places, including:
- Cracking from corrosion under insulation (CUI) which contains small amounts of chloride or where chlorides are present in the atmosphere;
- When a process is inadvertently contaminated with chlorides by unsuspecting people;
- Equipment that is is hydrotested with chloride contaminated water and left to dry out (concentrating the chlorides into small pools of highly aggressive salt solutions), which causes cracking on startup;
- Stainless steel deadlegs which collect chloride contaminated water;
- Instrument tubing that is normally not welded but contains high residual stresses comes in contact with chloride contaminated atmospheres; and
- Stainless steel bellows which typically have high stress levels come in contact with chloride contaminated environments especially during down time.
Image Gallery
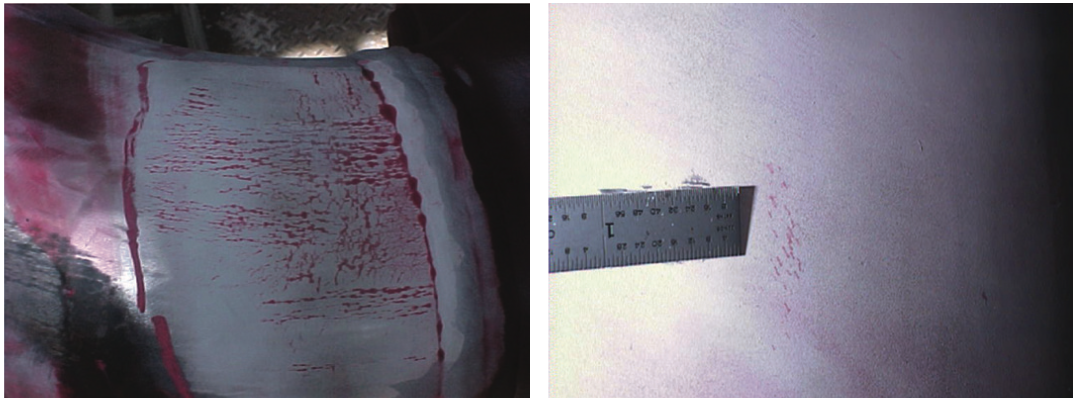
Corrosion Fatigue
| Foundational Concepts |
| Fatigue |
Corrosion fatigue is closely related to mechanical and vibration fatigue cracking, except that it is initiated and accelerated by a corrosion mechanism, especially one that gives rise to pitting, from which cracks often initiate. But that corrosion mechanism need not be very severe in order to give rise to corrosion fatigue.
Ethanol Stress Corrosion Cracking
| Foundational Concepts |
| Stress Corrosion Cracking |
Ethanol SCC is caused by the combination of a fuel grade ethanol (FGE) and, like all other forms of SCC, a tensile stress. This damage mechanism has been discovered in carbon steel storage tanks, rack piping, and associated equipment, and in FGE pipelines. It can be prevented or reduced through proper PWHT or through the usage of coatings.
Hydrogen Embrittlement
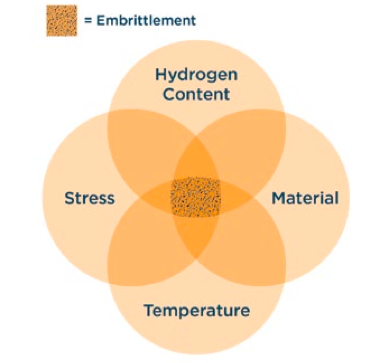
Hydrogen embrittlement is a potential threat for most carbon steels and Cr-Mo low-alloy steels exposed to services that can saturate the metal with atomic hydrogen. Hydrogen embrittlement has been observed in some stainless steels (400-series, precipitation hardening and duplex stainless steels, as well as in severely cold worked austenitic alloys such as the 300-series stainless steels). The affected metal, normally considered to be ductile, can fracture in a brittle manner.
Hydrogen embrittlement is a very simple, yet often misunderstood, mechanism. Atomic hydrogen is dissolved in the iron lattice taking up space. When the iron is hot, and therefore more ductile, the hydrogen present does not cause a problem. However, when the iron is cool, the hydrogen prevents the iron lattice from moving. This is referred to as embrittlement. It is then de-embrittled when the iron is heated and once again becomes ductile.
In general, hydrogen embrittlement is an insignificant threat for most services and normally not a threat above about 300°F (150°C). However, embrittlement may be a risk during shutdowns and downtime. During shutdowns, inspectors should avoid activities such as hammer testing and using potentially embrittled components for internal navigation. High strength low alloy bolting of trays and tray supports are examples.
The primary mitigation measure for suspected hydrogen embrittlement is to bake-out the hydrogen before initiating post-shutdown procedures.
Image Gallery
Hydrogen Stress Cracking - HF
Hydrogen stress cracking occurs when corrosion from acids like wet hydrogen sulfide or hydrofluoric acid (HF) cause atomic hydrogen to penetrate hardened or higher strength steels and cause stress cracking. Steel hardness, strength and stresses present are the critical factors determining susceptibility. When hydrogen stress cracking is a threat, equipment is often preheated and/or post weld heat treated (PWHT) to reduce hardness and residual stress levels.
Because hydrogen stress cracking is nearly always a surface phenomena, most any typical surface NDE is sufficient to find such cracking, and it’s not unusual for the cracks to be clearly visible to the naked eye, especially those that are transverse across a weld cap. Hardness testing can find welds and bolting material that will likely be more susceptible to hydrogen stress cracking.
Liquid Metal Embrittlement
Liquid Metal Embrittlement (LME), also referred to as Liquid Metal Cracking, occurs when molten metals come in contact with susceptible materials. Cracking rates can be exceedingly rapid and failure can occur within seconds. LME most commonly afflicts austenitic stainless steels, but can afflict other copper, nickel, and aluminum alloys. Damage resulting from LME appears as brittle cracks in an otherwise ductile material.
During a fire, molten metals may drip onto a susceptible metal, leading to LME. Liquid mercury contamination of some crude oils can also cause LMC in crude overhead condensers as well as the overheads of depropanizers and debutanizers. Mercury has also cracked aluminum core exchangers in ethylene plants.
For mitigating LME, any stainless steel that will be used in furnaces or a temperatures over about 780 F should not be allowed to come in contact with anything that has been galvanized or coated with zinc containing coatings. For detecting cracks, use MT for ferritic steel and PT for 300 Series SS and nickel base alloys. Radiography can be used to locate mercury deposits inside heat exchanger tubes.
Polythionic Acid Stress Corrosion Cracking
| Foundational Concepts |
| Stress Corrosion Cracking |
Polythionic Acid Stress Corrosion Cracking (PASCC) is an affliction of many refineries processing sulfur containing feedstocks. PASCC occurs when sensitized stainless steels that have been in service develop intergranular cracks after exposure to air and moisture, often during shutdowns. It typically does not occur during normal processing, but after equipment is shut down and opened up for inspection, i.e. when moist air contacts the surface of equipment that has been exposed to sour hydrocarbons in service.
Certain types of 300 series stainless steels that are exposed to elevated temperatures between 800 F (427 C) and 1700 F (927 C) during manufacture, fabrication (especially welding), or even in process environments (like furnace tubes), can become sensitized, which means their crystalline structure changes such that they become susceptible to intergranular corrosion. PASCC is just one type of intergranular corrosion that affects sensitized stainless steels. The commonly used types of stainless steels, 304/304H and 316/316H are particularly susceptible, whereas the stabilized grades like 321/347 and low carbon grades 304L/316L are much less susceptible.
A common form of prevention is to do a soda ashwash of the equipment before or right after it is exposed to air and moisture, e.g. opened for inspection during a shutdown (refer to NACE RP0170 for neutralization guidelines).
Adequate checks and QA/QC are required to make sure that one does not inadvertently install sensitized equipment.
PASCC can be found with penetrant testing. Welds are typically most susceptible because welding heating and cooling rates can cause significant alloy sensitization.
Sulfate Stress Corrosion Cracking
| Foundational Concepts |
| Stress Corrosion Cracking |
Sulfate SCC can occur with copper alloys that are in sulfate solutions. This type of cracking is associated with tubes in heat exchanger bundles in overhead distillation systems where sulfates can form. Some copper alloys are highly susceptible; however, the 90/10 and 70/30 copper nickel alloys are more resistant.
Eddy Current or visual inspection techniques can be used to inspect heat exchanger tubes for this type of cracking. Also, physical bending of sample tubes can detect early-stage Sulfate SCC cracks.
Wet H2S Damage (Blistering/HIC/SOHIC/SSC)
Wet H2S Damage is a common problem that can occur when carbon steel equipment becomes exposed to wet H2S service environment. There are a variety of forms of Wet H2S Cracking, including Hydrogen Induced Cracking (HIC) and Stress-Oriented Hydrogen Induced Cracking (SOHIC).
This damage mechanism can be a particularly dangerous form of corrosion because (1) damage caused by it takes place on the interior of vessels, (2) it can occur without warning, and (3) it can only be detected using complicated inspection methods.
Wet H2S Damage occurs due to the effects of aqueous hydrogen charging of steel in wet H2S process environments. This process can happen at relatively low temperatures, largely as a result of atomic hydrogen from wet H2S corrosion reactions which enter the steel and collect at inclusions or impurities within the steel. This happens because the H2S prevents the hydrogen recombination reaction that would normally occur, forcing the hydrogen atoms into the metal structure, leading to corrosion and weakness.
Wet H2S damage primarily occurs under acidic conditions, which are present in most oil refining environments. Any equipment that runs in conditions that are both above 50 ppm of H2S content and below 180F temperature in aqueous sour waters is likely susceptible to wet H2S cracking.
Hydrogen Induced Cracking (HIC) is a form of tiny blistering damage caused by a high concentration of hydrogen in steel. The blistering damage tends to form parallel to the surface and to the direction of hoop stress. Because of this, it usually doesn’t become damaging until it either becomes extensive and affects material properties, or gives rise to cracking that propagates into a weld or begins to go stepwise through the wall. On the surface, HIC is often horseshoe shaped and no bigger than the cuticle of one’s small finger.
Stress-Oriented Hydrogen Induced Cracking (SOHIC) is much more insidious. SOHIC is made up of a series of HIC cracks that are stacked perpendicularly in the direction of through wall cracks and driven by high residual or applied stresses. Because this damage can easily lead to integrity failures, facility owners should take measures to prevent or mitigate it when possible.
Sulfide Stress Cracking (SSC) occurs at locations where atomic hydrogen is able to diffuse at sites of high internal stress, such as grain boundaries, inclusions and regions of triaxial stress at notches. When placed in proximity to tensile stresses, embrittlement and the beginnings of brittle fracture may occur.
The most common NDE method for detecting wet H2S cracking is Wet Fluorescent Magnetic Particle Inspection (WFMP). This method is able to detect subsurface cracks in the steel that are caused by HIC, SOHIC, and SSC. For cracked piping and other components which cannot be inspected using WFMP, an alternative technique is Phased Array Ultrasonic Testing (PAUT).
Although detection is important, new stainless alloys can be implemented to replace traditional steels in applications where corrosion can be particularly severe. When coupled with chemical inhibitors, these alloys are effective at mitigating corrosion, although they may in some cases still be susceptible to SSC.
Equipment that is specifically susceptible to SOHIC can be made more resilient by incorporating post weld heat treatment (PWHT) and/or by being alloyed up. HIC-resistant steels and polymeric coatings have also been successfully used to prevent damage. In more aggressive environments, another solution might be using stainless steel clad materials, as they are more resistant to this sort of damage.
















