High Temperature Corrosion
Carburization
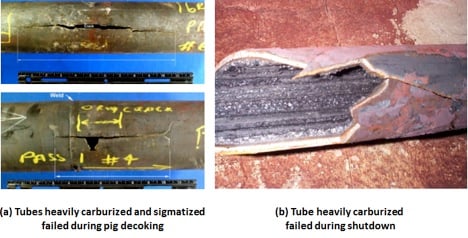
Carburization occurs when carbon is absorbed into a material at high temperature operating environments – typically temperatures above 1,100 F (593 C). Susceptible materials include carbon steel and low alloy steels, 300 and 400 Series stainless steels, cast stainless steels, some nickel base alloys, and HK/HP alloys. In order for carburization to occur, these materials must have exposure to a carburizing environment or carbonaceous material.
Carburization occurs most commonly in fired heater tubes but can also be found elsewhere, so long as the conditions above are met.
Prevention of this damage mechanism comes down to alloy selection or reducing the carbon activity in the environment through lower temperatures or higher oxygen/sulfur partial pressures.
Image Gallery
Decarburization
Decarburization is the antithesis of carburization and rarely results in equipment failure. However, surface decarburization is often a sign that something more serious is going on, namely high temperature hydrogen attack (HTHA). Decarburization can also be indicative of improper post weld heat treatment and help identify equipment that is fire damaged, as hardness testing will reveal a softening of the surface, indicative of loss of carbon (iron structure without carbon is relatively soft compared to steels with carbides in their structure).
Fuel Ash Corrosion
Fuel Ash Corrosion (or “catastrophic oxidation”) can occur when certain contaminants are present in a high temperature environment, for example inside furnace fireboxes. Those contaminants are typically vanadium pentoxide with sulfur oxide or sodium sulfate. When these contaminants are present in the combustion atmosphere, liquid slags can form on components operating above 1000F (538C) which can cause exceedingly high rates of corrosion (sometimes up to 1000 mpy).
There are a variety of methods to reduce the risk of fuel ash corrosion, including:
- blending or changing fuel sources (minimizing the contaminants) and operating equipment so that hot components are below the temperature where molten deposits are formed
- Proper burner design and burner management to reduce flame impingement and localized hot spots
- Firing with low excess oxygen or injecting special additives into the fuel, so as to increase the melting point of slags.
- In some components such as tube hangers and supports, changing to a 50%Cr-50%Ni alloy such as Alloy 657 may minimize corrosion.
Metal Dusting
Metal dusting is a severe form of carburization in which the extensive carbides that form as a result of carburization lead to grains of metal falling out of the tube or piping and being swept away by the process stream. This leaves a heavily pitted looking structure along with thinning of the tube or pipe. Sometimes those pits are still filled with a granular deposit of carbides that are easily chipped away during inspection and testing.
Nitriding
Above certain temperatures, process streams containing nitrogen compounds such as ammonia or cyanides will form a hard, brittle surface layer on some alloys – a metallurgical change known as Nitriding. Nitriding begins at temperatures above 600 F (316 C) and becomes severe above 900 F (482 C). Other critical factors are time, the partial pressure of nitrogen, and the alloy composition.
Carbon steels, low alloy steels, and 300/400 Series SS are affected by Nitriding. Alloys containing 30-80% nickel are more resistant. Therefore, mitigation may be achieved by changing to alloys with higher nickel content where it makes economic sense to do so.
Oxidation
All metals oxidize, even at room temperature, and in many cases that slow oxidation process actually protects the metal from rapid oxidation. Even rusting is a low temperature oxidation process. But at higher temperatures, oxidation can proceed fast enough to produce excessive scaling and thereby inhibit the usefulness of steels and alloys at elevated temperatures. For carbon steel the oxidation temperature limit is usually in the vicinity of 900F (482C) - 950F (510C) range.
For higher temperature oxidation resistance, as well as strength, steels are alloyed with chromium and molybdenum to increase their usefulness in high temperature applications such as furnace tubes, furnace outlet piping, hot hydroprocess equipment and catalytic reaction equipment.
Infrared thermography and surface thermocouples are two widely used methods of monitoring for temperatures that may exceed design conditions of construction materials. When high temperature oxidation failures do occur, it’s usually because the excessive scaling or hot spots are not obvious. They may be on furnace tubes that are not within sight of a furnace view port or may be occurring underneath external insulation. Refractory linings can fail, causing steel pressure retaining construction materials to be exposed to excessive temperatures. If the OD is insulated, these excessive temperatures may not be apparent.
Sulfidation
Sulfidation (or sulfidic corrosion) is corrosion that occurs with carbon steel and other alloys as a result of the presence of sulfur compounds in high temperature environments. It is a very common damage mechanism in the refining industry and has been the cause of several high profile incidents.
Sulfidation is typically of concern in sour (high in hydrogen sulfide or other active sulfur compounds) crude oil services starting at temperatures in the 500 F (260 C) range.
Common process units of concern are the crude, FCC, coker, vacuum, visbreaker, and hydroprocessing units. Heaters fired with oil, gas, coke, and most other sources of fuel may be affected depending on sulfur levels in the fuel. Boilers and other high temperature equipment exposed to sour gases may also be affected.
Sulfidation corrosion appears most often in the form of uniform thinning but may also occur as localized corrosion or high velocity erosion-corrosion damage.
Industry failures from sulfidation have occurred, in large part, for one of three reasons:
- Lack of adequate PMI, meaning that an inadvertent substitution of carbon steel or lower chromium alloy caused a component to fail prematurely or unexpectedly
- Piping systems having a mixture of higher silicon containing steels (“silicon-killed”) and lower silicon containing steels (“non-killed”).
- Process Creep, or the gradual increase over time in hydrogen sulfide content of process streams introduced by changing feedstocks.
Adequate PMI and MOC work processes can help to prevent unexpected piping failures from sulfidation.
















