Introduction
Gasification processing technology emerged in the early 1970s.[1-4] Different gasification technologies have been developed by Texaco, Dow, Shell, and Krupp-Kopper.1 The main purpose of gasification technology is to convert hydrocarbon products into Synthesis Gas (Syngas), mainly composed of hydrogen, carbon monoxide, and carbon dioxide to produce high combustion exothermic energy with better environmental performance.[1-5] Such energy is typically used for electrical power generation, via high-pressure steam production. The feedstock for gasification is diverse and includes biomass, coal, refining vacuum residuals, and fuel oil. This diversity presents a challenge for in the area of material selection, inspection, and corrosion control, especially due to the lack of historical experience with equipment degradation in these processes.
Many operators reported erosion and corrosion problems at their gasification plants, most of which were reported in pilot units. These include coal slurry erosion and corrosion in the gasification feed piping system, erosion and corrosion in gasification reactor refractory systems, high temperature corrosion in gasification effluent coolers, and aqueous corrosion in cooler downstream equipment.[1-3,6,7]
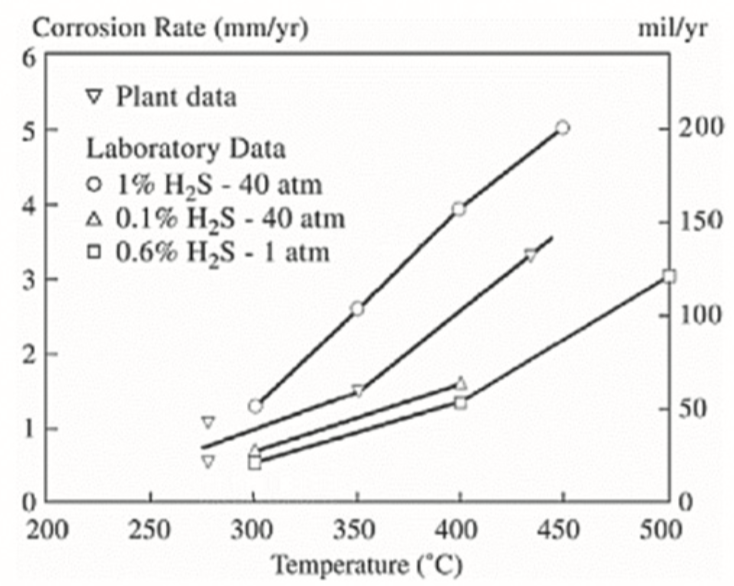
Bakker, in his review, analyzed the corrosion performance of low and high alloy steels and nickel alloys in gasification reactors and effluent coolers.[1] In his review, the sulfur, chlorine, and oxygen content in the raw Syngas played a significant role in the increase in corrosion rates observed in the gasification effluent coolers. In addition, the main corrosion damage mechanism observed in the gasification effluent cooler was sulfidation. In his work, a collection of corrosion data and material performance handling wet raw Syngas with the following volumetric composition ranges of 35-45% CO, 10-15% CO2, 27-30% H2, 15-25% H2O, 0.2-1.2% H2S and 50-600ppm HCl were analyzed. In addition, dry Syngas was included in his evaluation where CO reached a range of 62-64% and CO2 and H2O reached 4%. Figure 1 illustrates the corrosion increase in corrosion rates of low alloy steel effluent coolers as a function of operating temperature, pressure, and H2S content in the raw Syngas. Increasing the operating temperature, pressure and H2S content were observed to significantly increase corrosion rates, predominantly due to sulfidation. Figure 1 represents the corrosion rate increase when the feedstock has high coal composition and when the sulfur partial pressure is significantly higher than oxygen partial pressure.



















Comments and Discussion
Add a Comment
Please log in or register to participate in comments and discussions.